Novel way to quantify the thermal and non-thermal effects in plasmon-mediated chemical reactions
Plasmonics has drawn a lot of researchers’ attention for its huge application potential in enhanced spectroscopy (a way to obtain structural information down to even the single-molecule level), photothermal therapy, photoelectrocatalysis, photovoltaic devices, sensing, and optical waveguide. Recently, a research team led by scientists from City University of Hong Kong (CityU) has identified a novel way to decouple and quantify the thermal and non-thermal effects shown in plasmon-mediated chemical reactions. The team could even control the two effects precisely, which is important for designing and optimizing plasmonic devices and promote their applications.
Their research findings were published in the scientific journal Angewandte Chemie, titled “Thermal and Nonthermal Effects in Plasmon-Mediated Electrochemistry at Nanostructured Ag Electrodes”.
Conventional technique: inaccurate temperature measurement
Surface plasmons are the resonant oscillations of the free electron on the surface of nano-metals when stimulated by light. Metals exhibit special photoelectric properties in a resonance condition, such as a huge increase in surface potential. This provides the possibility to manipulate the interaction between light and matter in a nanoscale. Therefore plasmonics has drawn much attention in the fields of both basic research and applied research.
Most plasmonic applications are closely associated with the non-radiative decays of plasmons, where hot carriers (refers to electrons and electron holes that have gained very high kinetic energy) are generated, causing the hot carrier effect (non-thermal effect). And at the same time, local heating (thermal effect) will occur.
Quantifying both the thermal and non-thermal effects will help in optimizing the efficiency of the plasmonic devices. The conventional measuring method goes like this: the thermal effect is first calculated based on the surface temperature change recorded by a thermal camera, scientists would then subtract the thermal effect from the overall plasmonic effects to deduce the contribution of non-thermal effect.
Unfortunately, the accurate measurement of the surface temperature at the reaction sites remains an exceptionally difficult task for current techniques, and even a small error may result in a completely different conclusion. As a result, scientists cannot reach a consensus on which effect is the dominant one.
The research team led by Professor Lu Jian, CityU’s Vice President (Research and Technology) and Director of the Hong Kong Branch of National Precious Metals Material Engineering Research Center, has dug deep into this issue and achieved a breakthrough. Bypassing the difficulty of measuring the surface temperature, the team came up with a novel method to quantify precisely the contribution of thermal and non-thermal effects.
Researchers first designed an experimental setup for observing the photoelectrochemical behaviours of the plasmonic silver electrode. The lower part of the silver needle was electrochemically treated so that uniform nanospheres which supported plasmonic excitation were formed on the surfaces. Laser beam was then illuminated at three different sections of the silver electrode. Researchers observed the photocurrents resulted and had the following discoveries:
1)No noticeable current variation was resulted when the laser beam was illuminated at the untreated part of the silver electrode, which was above the liquid level of electrolyte;
2)Slow-response currents (ISC)were observed when the laser beam was illuminated at the section with nanospheres and above the liquid level of electrolyte;
3)Rapid-response currents (IRC)and slow-response currents were both observed when the laser beam was illuminated at the section with nanospheres and immersed in electrolyte (simulating the typical plasmon-mediated chemical reactions setting).


Dr Li Yangyang, Associate Professor at CityU’s Department of Materials Science and Engineering who led the experiment, explained that the above three totally different responses reveal distinct response pathways. In particular, when the laser beam is illuminated at the nanostructured silver electrode which is above the liquid level of electrolyte, hot carriers generated from plasmon-mediated chemical reactions are unable to reach the reaction sites in the electrolyte, because of their shorter transportation range. Therefore rapid-response currents cannot be formed.
The obvious contrast has confirmed that the slow-response currents observed on the nanostructured silver electrode in the third scenario (simulating the typical plasmon-mediated chemical reactions setting) are resulted from the thermal effect. And the rapid-response currents generated on the illuminated nanostructured silver electrode in the electrolyte, are resulted from the non-thermal effect.
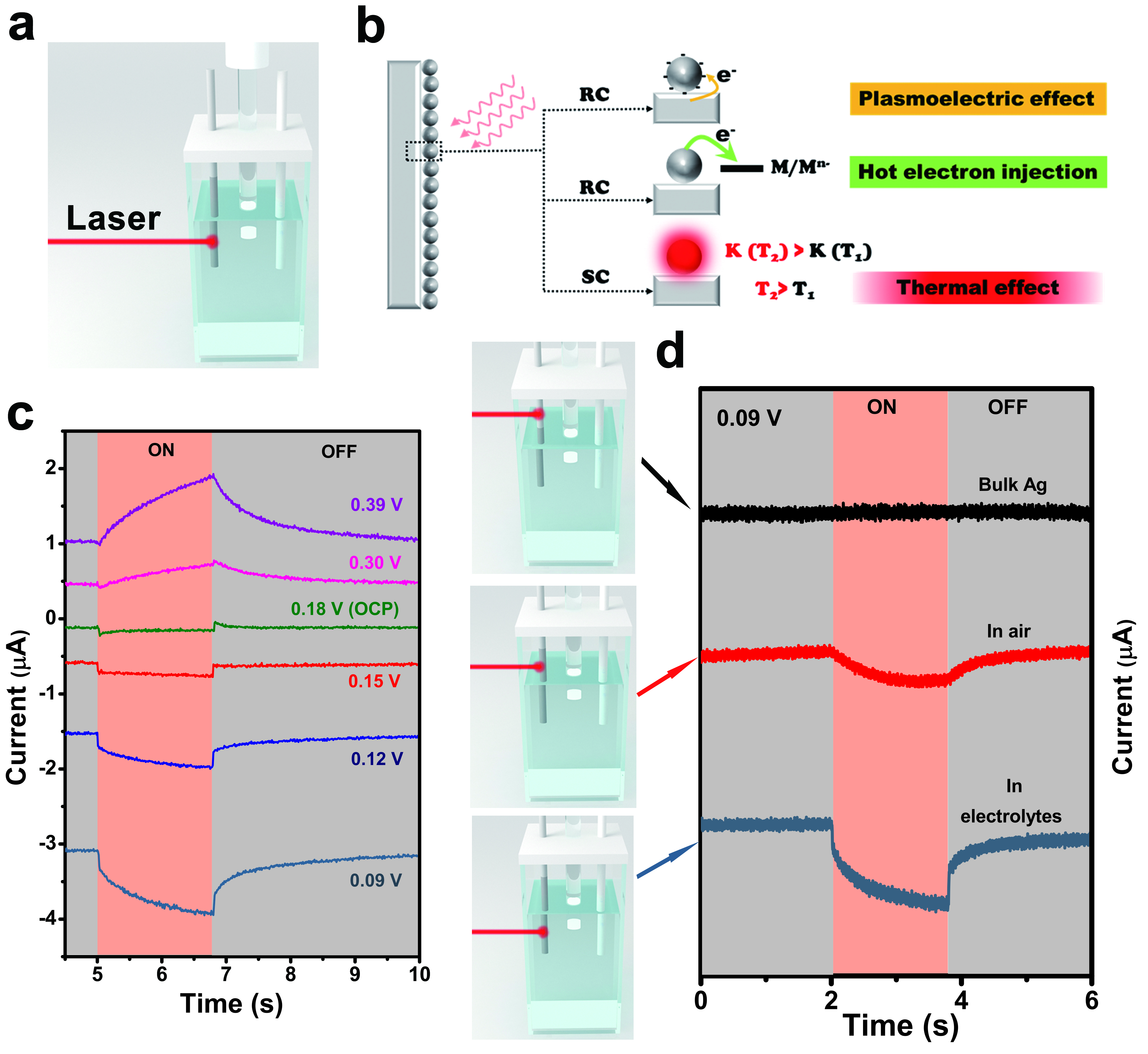
The team further investigated the changes of rapid-response currents, slow-response currents, and the total photocurrents at different electrode voltage in electrolyte with laser illumination switched on and off. In this way, the team quantified the thermal and non-thermal effects exhibited in plasmon-mediated chemical reactions. And for the first time, researchers found that plasmoelectric surface potential contributed to the rapid-response currents.

Controllable contribution ratio of thermal and non-thermal effects
Researchers also discovered that slow-response currents have shown an increase at higher electrode bias, and were always larger than rapid-response currents except at 0.15 V. These results indicated that the thermal effect played a dominant role with larger contribution compared with non-thermal effects in the plasmon-mediated chemical reactions in the system. Furthermore, the ratio between thermal and non-thermal effects could be manipulated by adjusting the electrode potential, laser wavelength, and intensity.
Last but not least, researchers were able to derive the actual temperature of reaction sites from the data of slow-response currents. They discovered that the temperature increment turned out to be 6 times larger than the readings recorded from a conventional thermal camera. They believed that the contribution of thermal effects might be underestimated in previous studies.
“Through bypassing the difficulty of temperature measurements, our team has provided a reliable and convenient method for quantitatively evaluating the thermal and non-thermal effect contribution in plasmon-mediated electrochemical reactions based on photoelectrochemical behaviours. It is also the first time to quantitatively identify the contributions of plasmoelectric surface potentials to rapid-response currents. We could even control the contribution ratio of thermal and non-thermal effects. This would undoubtedly shed some light on plasmon-mediated electrochemical reactions and promote its application,” concluded Professor Lu.
Professor Lu and Dr Li Yangyang, both from CityU’s Department of Materials Science and Engineering, are the corresponding authors of the paper. The first authors are Dr Ou Weihui and Dr Zhou Binbin, both from the same department. The CityU research team members included Associate Professor Dr Lei Dangyuan, Senior Research Associate Dr Li Shengliang, PhD student Shen Junda, and Zhong Jing, all from the Department of Materials Science and Engineering. Dr Lo Tsz Wing from the Department of Applied Physics of The Hong Kong Polytechnic University is also one of the research team members.


The study was supported by the National Key Research and Development Program of China, the Major Program of the National Natural Science Foundation of China, as well as the Innovation and Technology Commission of HKSAR.
DOI number: 10.1002/anie.202001152