
A research team led by Professor Paul Kim-ho Chu, a materials engineering expert at City University of Hong Kong (CityU), has developed a capacitive coating that kills bacteria when it is charged with electricity. When applied to orthopedic implants such as artificial joints and dental implants, the novel technology can reduce the risk of infection after surgery and help patients recover more quickly.
Professor Chu, Chair Professor of Materials Engineering in the Department of Physics and the Department of Materials Science and Engineering, and his research team discovered that titanium oxide (TiO2) nanotubes doped with carbon (TNT-C) could kill bacteria continuously for a period of time after charging with a small electrical current. Their research has been published recently in the international journal Nature Communications.
In their experiments, the team applied both direct and alternating current (DC, AC) to explore the post-charging antibacterial effects of TNT-C samples predesigned with different capacitances. They discovered that different TNT-C samples showed different antibacterial efficiency during charging, with TNT-C-15 being the most effective in killing Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) (Fig. 1b). More importantly, the charged samples continued to kill bacteria after the DC+ power had been turned off (Fig. 1c) and the positive DC (DC+) charging mode showed the best antibacterial effect.
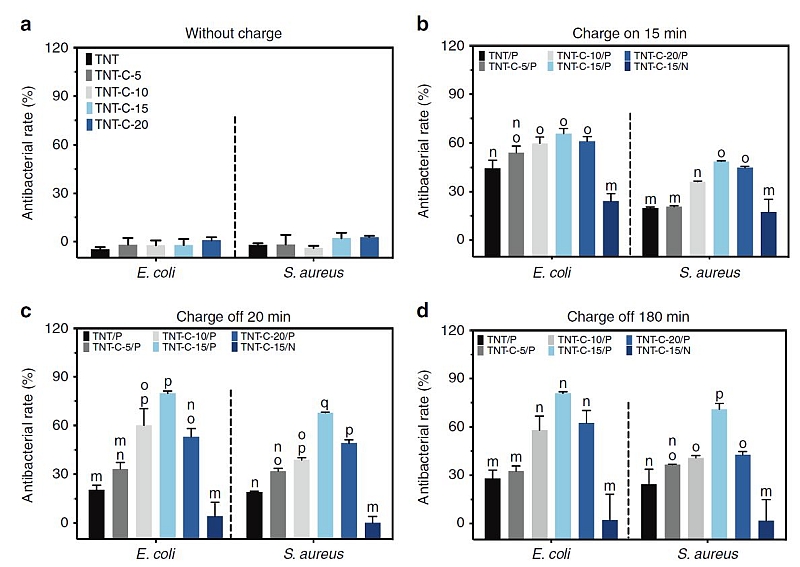
To confirm the universal antibacterial effects, TNT-C-15 was further tested with two additional strains of bacteria commonly found in the oral and respiratory environment, namely Staphylococcus epidermidis and Pseudomonas aeruginosa.
It was found that by means of cyclical DC+ charging, TNT-C-15 could achieve a bacterial killing efficacy of over 90% for all the four genera of bacteria, thus boding well for clinical application (Fig 2).
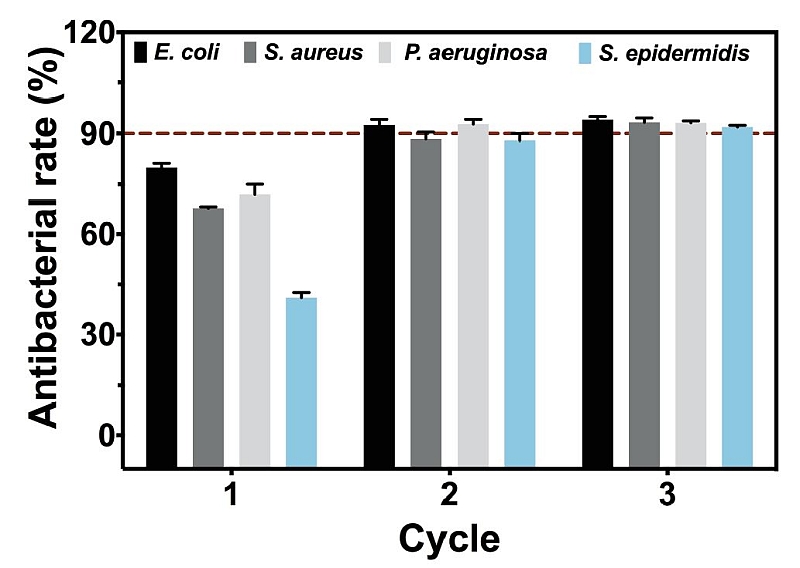
Moreover, the team revealed that through cyclical charging, the capacitance-based platform could effectively prevent the formation of biofilms that might otherwise cause resistance to medicines like antibiotics.
According to Professor Chu, using a high voltage to kill bacteria is not new, but what makes this a breakthrough is that the antibacterial effect can be achieved with charging and the effect can last for more than five hours after each charging cycle. TNT-C-15 can kill bacteria after being charged for 15 minutes with a small direct current of 2 volts or less and the effect can be maintained for over several weeks if the coating is repeatedly charged.
The positive electrode on the surface of the nanotube coating leads to extracellular electron transfer (EET) from the bacteria to the charged TNT-C, which impairs the cell membranes of the bacteria and induces reactive oxygen species (ROS) burst in bacteria. These events kill bacteria, but the growth and normal functions of osteoblasts are not affected.
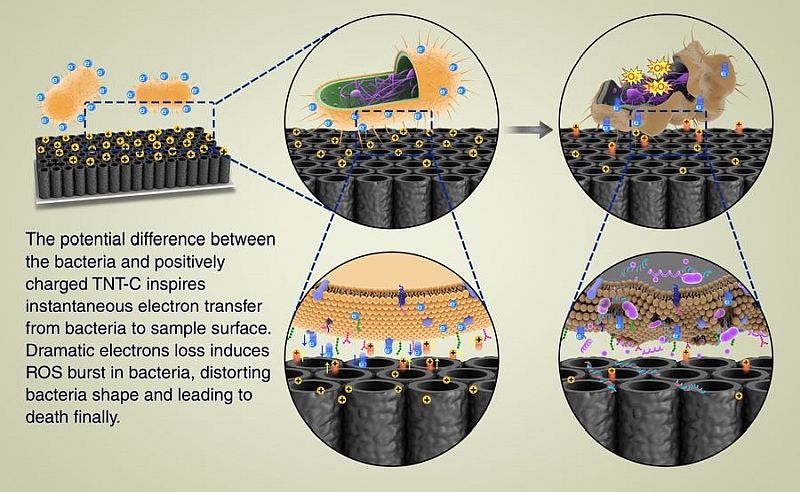
Professor Chu believes that by adding such a capacitive coating to orthopaedic implants like artificial joints or dental implants together with a built-in micro charging device or through wireless charging, the new technology can protect patients from infection after surgery. Throughout the healing process, the technology does not produce any harmful side effects in patients and charging can stop after the patients have recovered.
“We are very excited about the research outcome,” Professor Chu said. “Currently, patients have to take medication such as antibiotics in the early post-surgery stage to prevent bacterial infection. However, problems such as drug resistance or other side effects may develop. This new technology of charging TNT-C is totally biocompatible and does not have any side effects, and the coating can retain electrical charges to kill bacteria for an extended period of time. This groundbreaking technology can reduce complications arising from bacterial infection and improve the well-being of patients.”
With clinical trials to follow, he hopes that the technology will be applied to medical treatment in the future.
The discovery was published in Nature Communications in May under the title “An antibacterial platform based on capacitive carbon-doped TiO2 nanotubes after charging with direct or alternating currents”.
This research article originated from CityU Research Stories.