- 1A-405, 4/F, Block 1, To Yuen Building
- +852 3442-4152
- +852 3442-0128
- kwokolai@cityu.edu.hk
- CityU Scholars
- Lab Website
Prof. Kwok-On Lai received his Bachelor degree of Biochemistry with First Class Honours from the University of Hong Kong. He then pursued M.Phil and Ph.D studies at the Hong Kong University of Science and Technology (HKUST) under the guidance of Prof. Nancy Ip. With the award of Croucher Foundation Fellowship, Prof. Lai performed postdoctoral research in the United States with Prof. Kelsey Martin at UCLA, where he used photoactivation and time-lapse imaging to study synapse-to-nucleus protein trafficking in neuron that underlies long-term synaptic plasticity. Prof. Lai returned to Hong Kong and joined HKUST as Research Assistant Professor to investigate the role of protein phosphorylation in synaptic plasticity and memory formation. He then set up his own laboratory at the University of Hong Kong, where his team studies the interplay between intracellular transport, protein post-translational modification and synaptic function of neuron. His recent research includes the investigation of disease-associated genetic variations using human stem cell-derived neuron. Prof. Lai joined City University in 2021.
Research Interest
Neurons communicate with each other through neurotransmission at synapses. To support the specific functions of the synapses, different molecules must be delivered properly from the cell body to the presynaptic axon terminals and the postsynaptic dendritic spines. Consequently, disruption of intracellular transport may cause synaptic defects that impair brain functions including learning and memory. Knowledge about transport mechanism in neuron is therefore crucial for our understanding on the diseased brain. There are subsets of mRNAs in the cell body that can be transported along the microtubule to dendrites via interaction with multiple RNA-binding proteins and molecular motors. These mRNAs can be translated near synapses in response to synaptic activity, and this local protein synthesis is essential for the persistent changes in synaptic efficacy during memory formation. Advances in RNA sequencing allow the identification of many dendritically localized mRNAs, but most of their functions in the brain remain largely unexplored.
Our laboratory aims to unravel key signaling pathways that control synapse formation and plasticity via regulation of intracellular transport and cytoskeleton. We have recently identified arginine methylation of molecular motor and RNA-binding protein as crucial mechanisms in synapse development, and demonstrated a cross-talk between arginine methylation and actin cytoskeleton dynamics at the synapse. Using advanced imaging to visualize trafficking of molecules and synthesis of proteins, the team is currently investigating the dendritic transport mechanism of mRNAs, as well as the regulation of motor and RNA-binding proteins by post-translational modifications.
Another area of research in the laboratory is to delineate the mechanisms of inherited brain disorders. Through generation of mutant mice and animal behavioral study, we investigate the molecular basis of epilepsy causes by gene mutations. We also employ CRISPR to edit the genome of human induced pluripotent stem cells and uncover the effects of neurodegeneration-causing mutations on human neuron.
Another area of research in the laboratory is to delineate the mechanisms of inherited brain disorders. Through generation of mutant mice and animal behavioral study, we investigate the molecular basis of epilepsy causes by gene mutations. We also employ CRISPR to edit the genome of human induced pluripotent stem cells and uncover the effects of neurodegeneration-causing mutations on human neuron.
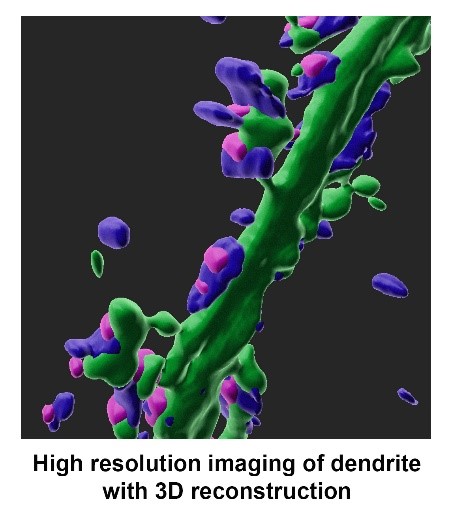
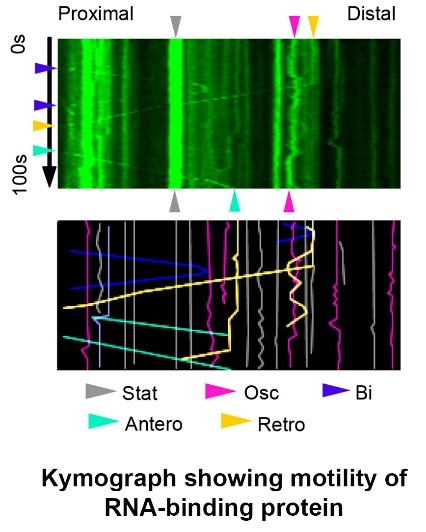
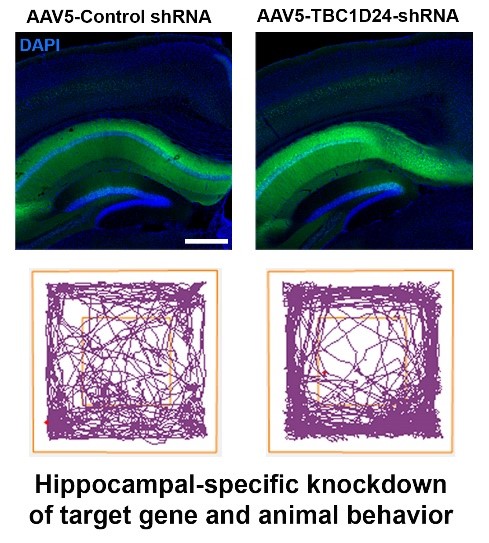
- Kinesin-mediated intracellular transport and RNA trafficking in neuron
- Post-translational control of RNA-binding proteins and local dendritic translation
- Genome editing in human stem cells to study brain disorders
- Synaptic defects in inherited epilepsy
Position Available
We are seeking for highly-motivated PhD students and Research Assistants to join our team. They should have strong passion in neuroscience or cell biology, and eagerness to employ new techniques and image analysis. Interested candidates please contact kwokolai@cityu.edu.hk.
Selected Publications
(* corresponding authors)
- Fok AHK, Huang Y, So BWL, Zheng Q, Tse CSC, Li X, Wong KKY, Huang J, Lai KO*, and Lai CSW* (2024). KIF5B plays important roles in dendritic spine plasticity and dendritic localization of PSD95 and FMRP in the mouse cortex in vivo. Cell Rep. 43: 113906. DOI: 10.1016/j.celrep.2024.113906
- Dong R, Li X, Flores A and Lai KO* (2023). The translation initiating factor eIF4E and arginine methylation underlie G3BP1 function in dendritic spine development of neurons. J. Biol. Chem. 299: 105029. DOI: 10.1016/j.jbc.2023.105029.
-
Lo LHY, Dong R, Lyu Q and Lai KO* (2020). The protein arginine methyltransferase PRMT8 and substrate G3BP1 control Rac1-PAK1 signaling and actin cytoskeleton for dendritic spine maturation. Cell Rep. 31: 107744. DOI: 10.1016/j.celrep.2020.107744.
Featured in Croucher Foundation News -
Zhao J, Fok AHK, Fan R, Kwan PY, Chan HL, Lo HY, Chan YS, Yung WH, Huang J, Lai CSW*, Lai KO* (2020). Specific depletion of the motor protein KIF5B leads to deficits in dendritic transport, synaptic plasticity and memory. eLife. 9: e53456. DOI: 10.7554/eLife.53456.
Featured in Research Highlights in Nature Reviews Neuroscience and eLife Digest -
Lin LF, Lyu Q, Kwan PY, Zhao J, Fan R, Chai A, Lai CSW, Chan YS, Shen X, Lai KO* (2020). The epilepsy and intellectual disability-associated protein TBC1D24 regulates the maintenance of excitatory synapses and animal behaviors. PLoS Genet. 16: e1008587. DOI: 10.1371/journal.pgen.1008587
Featured in Croucher Science Week Blog - Lin LF, Lo HY, Lyu Q, Lai KO* (2017) Determination of dendritic spine morphology by the striatin scaffold protein STRN4 through interaction with the phosphatase PP2A. J. Biol. Chem. 292: 9451-9464.
- Liang Z, Zhan Y, Wong CC, Yates JR 3rd, Plattner F, Lai KO, Ip NY (2016) The pseudokinase CaMKv is required for the activity-dependent maintenance of dendritic spines. Nat. Commun. 7:13282. doi: 10.1038/ncomms13282.
- Tong BC, Lee CS, Cheng WH, Lai KO, Foskett JK, Cheung KH (2016) Familial Alzheimer's disease-associated presenilin 1 mutants promote γ-secretase cleavage of STIM1 to impair store-operated Ca2+ entry. Sci. Signal. 9: ra89. doi: 10.1126/scisignal.aaf1371.
- Lai KO*, Liang ZY, Fei E, Huang H, Ip NY* (2015) Cdk5-dependent phosphorylation of p70 ribosomal S6 kinase (S6K) is required for dendritic spine morphogenesis. J. Biol. Chem. 290: 14637-14646
- Liang Z, Ye T, Zhou X, Lai KO, Fu AKY, Ip NY. (2015). Cdk5 Regulates Activity-Dependent Gene Expression and Dendrite Development. J. Neurosci. 35: 15127-15134. doi: 10.1523/JNEUROSCI.1443-15.2015.
-
Lai KO, Wong ASL, Cheung MC, Xu P, Liang Z, Lok KC, Xie H, Palko ME, Yung WH, Tessarollo L, Cheung ZH, Ip NY. (2012) Serine phosphorylation of TrkB by Cdk5 is required for activity-dependent structural plasticity and spatial memory. Nat. Neurosci. 15: 1506-1515.
Featured in News and Views of Nature Neuroscience, Editor’s choice of Science Signaling and Faculty 1000 Biology -
Lai KO, Zhao Y, Ch’ng TH, Martin KC (2008) Importin-mediated retrograde transport of CREB2/ATF4 from distal processes to the nucleus in neurons Proc. Natl. Acad. Sci. U.S.A. 44: 17175-17180.
Highlighted by the Faculty 1000 Biology
Invited Review Articles
- Fan R and Lai KO* (2022). Understanding how kinesin motor proteins regulate postsynaptic function in neuron. FEBS J. 289: 2128-2144. doi: 10.1111/febs.16285.
- Dong R, Li X, Lai KO*(2021). Activity and Function of the PRMT8 Protein Arginine Methyltransferase in Neurons. Life (Basel) 11: 1132. doi: 10.3390/life11111132.
- Lo LHY and Lai KO* (2020). Dysregulation of protein synthesis and dendritic spine morphogenesis in ASD: studies in human pluripotent stem cells. Mol. Autism 11: 40. DOI: 10.1186/s13229-020-00349-y.
- Shen X, Yeung HT, Lai KO* (2019) Application of Human-Induced Pluripotent Stem Cells (hiPSCs) to Study Synaptopathy of Neurodevelopmental Disorders. Dev. Neurobiol. 79: 20-35.
- Lai KO, Ip NY (2013) Structural plasticity of dendritic spines: The underlying mechanisms and its dysregulation in brain disorders. Biochim. et Biophys. Acta. 1832: 2257-2263.
Activities
- Hong Kong Institute for Advanced Study (HKIAS) Rising Star Lecturer for Life Sciences
- Chair of the Symposium “Towards an understanding of how the cytoskeleton and intracellular transport are regulated for synapse development and plasticity”; the International Society for Neurochemistry (ISN) Annual Meeting 2022
- Associate Chair in the Gordon Research Seminar (GRS) in Molecular and Cellular Neurobiology “Exploring the Frontiers of Foundational and Translational Neuroscience”
- Associate Editor of Frontiers in Molecular Neuroscience
- Editorial Board member of Scientific Reports and Frontiers in Molecular Neuroscience
- Lead Guest Editor for the Special Issue “Molecular Mechanisms of Dendritic Spine Development and Plasticity” in Neural Plasticity
- Ad-hoc reviewer for Cell Reports, EMBO Reports, Science Signaling, Stem Cells, iScience
1 July 2024
