
Cancer is a complex disease characterized by genetic mutations that accumulate over time. Detecting cancer at an early stage is crucial for effective treatment. Scientists have made significant progress in using a non-invasive method called circulating tumor DNA (ctDNA) to detect liver cancer. Circulating cell-free DNA-inferred tumor content has been demonstrated detectable previously in blood samples collected at diagnosis of early-stage cancer. However, limited data has been shown in samples collected prior to cancer diagnosis – “pre-sample”.
In a recent study published in Clinical Cancer Research, a research collaboration team from Prof. Zongli Zheng and Prof. Mingfang Ji of Zhongshan People’s Hospital developed a novel simple and robust DNA sequencing technology for low-pass genome-wide sequencing. Based on this method, they then evaluated tumor content in circulation throughout the natural history of hepatocellular carcinoma (HCC) development. The study was led by Shifeng Lian, M.D./M.Sc., a PhD candidate from Karolinska Institutet.
The results showed that high circulating tumor content was associated with advanced tumor stage (P < 0.001) and a poor survival after HCC diagnosis (hazard ratio 12.35). Circulating tumor content turned negative after surgery (P = 0.027, see Figure above for an example patient), while remained positive after transarterial chemoembolization treatment (P = 0.578).
In pre-HCC samples, tumor content increased from 0.014 in 4 years before diagnosis to 0.026 in 1 year before diagnosis. The sensitivity of circulating tumor content in detecting HCC increased from 22.7% within one year before diagnosis to 30.4% at The Barcelona Clinic Liver Cancer (BCLC) stage 0/A, 81.8% at stage B, and 95.5% at stage C (see Figure below). The specificity was 97.8%, which is important for future screening studies in high-risk populations to achieve a high positive predict value (PPV).
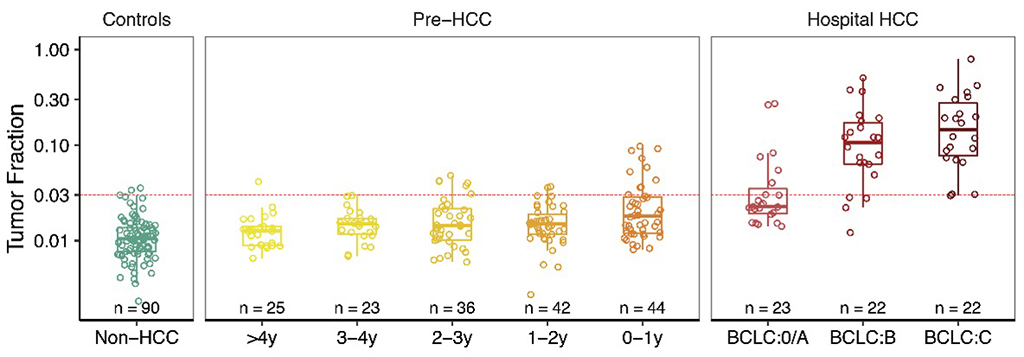
The findings suggest that monitoring circulating tumor content can provide valuable prognostic information and potentially enable the detection of hepatocellular carcinoma one year earlier than clinical diagnosis. This method shows promise for identifying asymptotic hepatocellular carcinoma in high-risk populations.